About Secretory Pathways
The primary route for protein secretion from eukaryotic cells is termed the classical, or endoplasmic reticulum (ER)/Golgi-dependent, secretory pathway. Briefly, secreted eukaryotic proteins utilize an N-terminal signal peptide (SP) to direct their co-translation on ER-bound ribosomes into the ER lumen, after which they progress through the endomembrane system and are ultimately exported to the extracellular environment, or cell surface. It should be noted that these represent a subset of SP-containing proteins, since others are retained in the ER or Golgi or are redirected to other compartments, such as the vacuole.
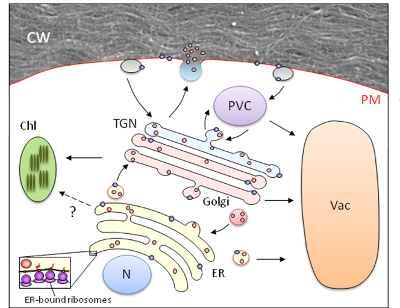
Schematic diagram of the plant classical secretory pathway, highlighting protein co-translational translocation from ER-bound ribosomes in the ER lumen and the subsequent major trafficking pathways (N, nucleus; ER, endoplasmic reticulum, Chl, chloroplast; TGN, trans-Golgi network; PVC pre-vacuolar compartment; PM, plasma membrane; CW, cell wall). Adapted from Foresti and Denecke (2008).
There is also growing evidence that plant proteins can be secreted to the apoplast via routes that are independent of the ER-Golgi pathway. Such proteins do not have canonical signal peptides and treatment with chemical inhibitors, such as brefeldin A, which disrupt vesicle trafficking between the ER and Golgi, and does not perturb secretion. While such non-classical pathways have been studied in yeast and mammalian cells, and a search tool is available for such mammalian proteins, essentially nothing is yet known about such pathways in plants. This will be addressed in this project.